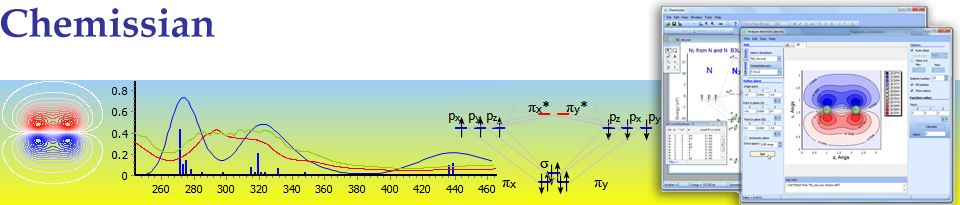
MO diagram for formation of nitrogen molecule from atoms - Atomic and Molecular Orbitals
The work of Chemissian molecular orbital editor is shown.
Chemissian provides ability to work with several molecular orbital (and Kohn-Sham) diagrams at the same time.
Presented diagram shows how the atomic orbitals of two N atoms combine to form molecular orbitals of nitrogen molecule: each nitrogen atom has three one-occupued p-orbitals and when the atoms interact they produce three bonding molecular orbitals of nitrigen molecule - one of sigma-type(symmetry) (from atoms pz) and two pi-type (from atoms px, py); no antibonding orbitals is produced. Thus nitrogen molecule has triple bond.
For building/drawing this MO diagram quantum-chemical calculations of N atom and N2 molecule have been performed using B3LYP method and STO-3G basis set. These output files were loaded into Chemissian and using molecular orbitals editor, program built the diagram. Also graphical objects (text labels, electrons and connector lines from draw tools tool window) were added directly on the diagram.
see also: